Company
Technology
Support

SwitchPlex™
T790M KitEGFR T790M Mutation test
About 85% of Lung cancer is Non Small Cell Lung Cancer (NSCLC), and 70% of NSCLC is caused by overexpression of epidermal growth factor receptor gene.
EGFR tyrosine kinase inhibitor (TKI) treatment significantly delays the progression of NSCLC, however, exon 20 T790M mutation is related to the acquired tolerance to TKI treatment. Therefore, patient prescribed with Gefitinib or Erlotinib needs regular monitoring of EGFR T790M mutation tests and patients with test positive for the mutation needs to change the prescription to Osimertinib.
1.
First-Line Treatment
(gefitinib, erlotinib)
Approx. 50% of patients develop resistance in 6 months
2.
After T790M test
Second-Line Treatment
3.


HB SwitchPlex™
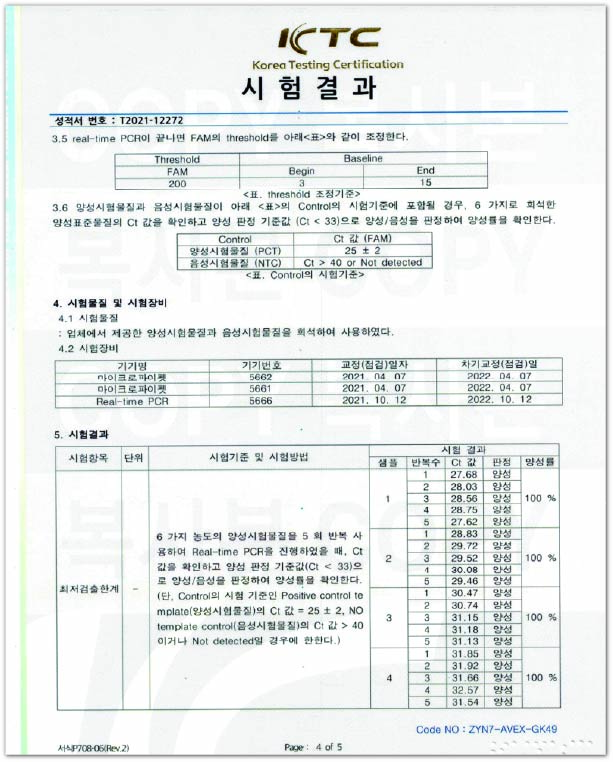
T790M KitL.O.D: 10⁰ Copies
Internationally certified Korea Testing Certification institute (KTC) confirmed the Limit of Detection (L.O.D) for HB SwitchPlex™ T790M Kit
Osimertinib
Mutation positive patients need proper prescription for targeted agents to treat the somatic mutation in early variation.
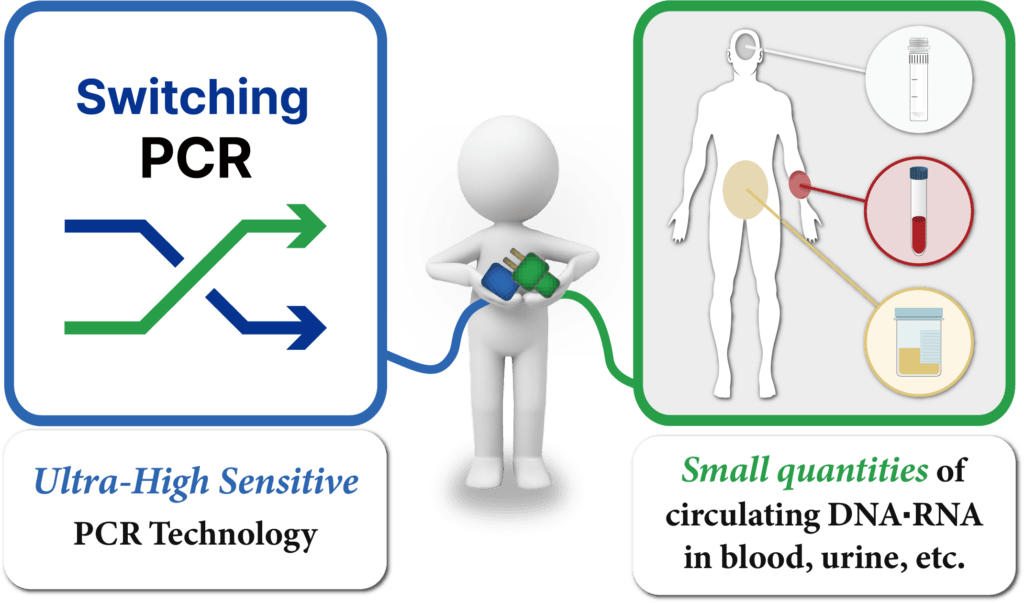

HB SwitchPlex™ products are applied with Switching™-PCR Technology which detects target genes in low concentrations with high reproducibility and accuracy
해당 제품의 관련한 기술인 HB Switching™ 페이지로 이동합니다.
No. 501, Innosphere
140, Gwacheon-Daero 12-Gil,
Gwacheon-si, Gyeonggi-do, South Korea, 13824
Business Registration No. : 135-86-48279
Tel : 1522-7026
Fax : 031-548-2135
E-mail : info@heimbiotek.com
