Products

miRDx™
EarlyHCC KitHCC Diagnostics Using miRNA Biomarkers
Intro
WHO reports that Liver Cancer has the fourth highest mortality rate in the world in 2022. The issue with cancer diagnostics is that current tests are effective after considerable disease progression and are either low in accuracy, costly or highly invasive.
Therefore, to reduce the Case Fatality Rate (CFR), a minimally invasive molecular diagnostic method that can accurately detect cancer in its early stages (1-2 stages) is essential.
HB miRDx™ EarlyHCC Kit is a reverse transcription RT-qPCR kit that detects circulating microRNA biomarkers for early stage (1~2) to late stage(3~4) HCC from blood.
Final ECT Results
Clinical Trial Status
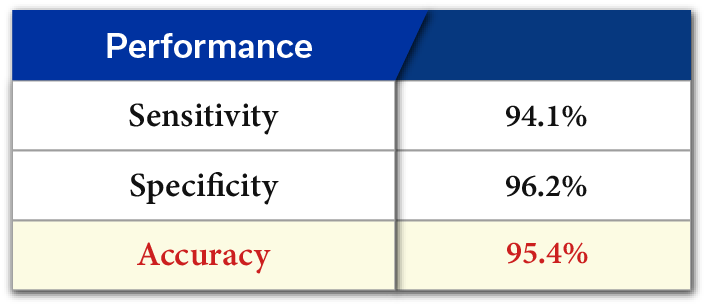
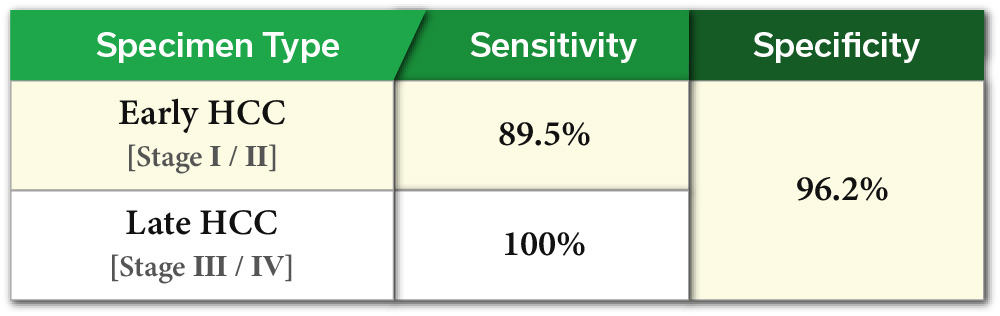
HB miRDx™ EarlyHCC Kit has completed Research Clinical Trials (RCT) and two Exploratory Clinical Trials (ECT) with approximately 500 clinical samples for an overall accuracy of 95.4%, sensitivity of 94.1% and specificity of 96.2%. The kit has a sensitivity of 89.5% for Stage 1~2, and 100% for Stage 3~4 liver cancer which demonstrates its potential as a widely utilized early liver cancer screening kit via liquid biopsy of blood.
Competitive Advantage : Key Benefits
HB miRNA Detection
for Early Cancer Diagnostics
1.
Sequencing
PCR product can be sequenced successfully to validate biomarkers for diagnostic kits. Location of target miRNA sequence is towards the middle of the PCR products unlike other commercial products.
2.
Multiplexing
Qualitative analysis with multiplexing for numerous clinical applications such as detection of infectious diseases during early stages or viral latency.
3.
Higher Reactivity
Precise hybridization during cDNA synthesis based on high reactivity enabling accurate detection and analysis.
4.
Reference miRNA System: circulating miRNAs
Detection of early cancer relies on quantitative analysis of circulating miRNA biomarkers in blood. The persistent need for identification of stable reference miRNA for normalization, resulted in the development of a reference miRNA system for circulating miRNAs to be used in liquid biopsy-based molecular diagnostics.
5.
qPCR Technology with Highest Sensitivity
HeimBiotek’s quantitative PCR platform demonstrates the highest sensitivity compared to other commercially available technologies. It is capable of detecting as little as 10⁰ copies of cDNA per reaction, thus facilitating the development of a wide range of miRNA biomarkers that has been difficult to develop duw to low sample volume
6.
RT-qPCR miRNA Detection Early Cancer Biomarkers
Only HeimBiotek Inc. possesses both miRNA detection and analysis IPs in addition to the capability of developing precise early cancer miRNA biomarkers.
Related Technology


miRDx™ Technology
HB miRDx™ is one of three miRNA detection technologies (RT-qPCR based) in the world. This technique possesses multiplexing capabilities and allows for accurate sequencing of PCR products, in addition to boasting high sensitivity and specificity.
해당 제품의 관련한 기술인 HB miRDx™ 페이지로 이동합니다.

