Products

SBDE-Plex™
Companion Dx KitPrevent Side Effects from Prescription Drugs
Intro
Drug side effects lead to significant socioeconomic expenses. In 2014, 430,827 people were diagnosed with drug side effects within South Korea resulting in medical expenses up to 273.8 billion Won. The sum of medical, transportation and nursing costs etc., in addition to the loss of income has been estimated to amount to 535.2 billion Won.
Prescription of universal anticancer drugs without taking genetic differences between patients into consideration, may lead to drug side effects besides economic loss.
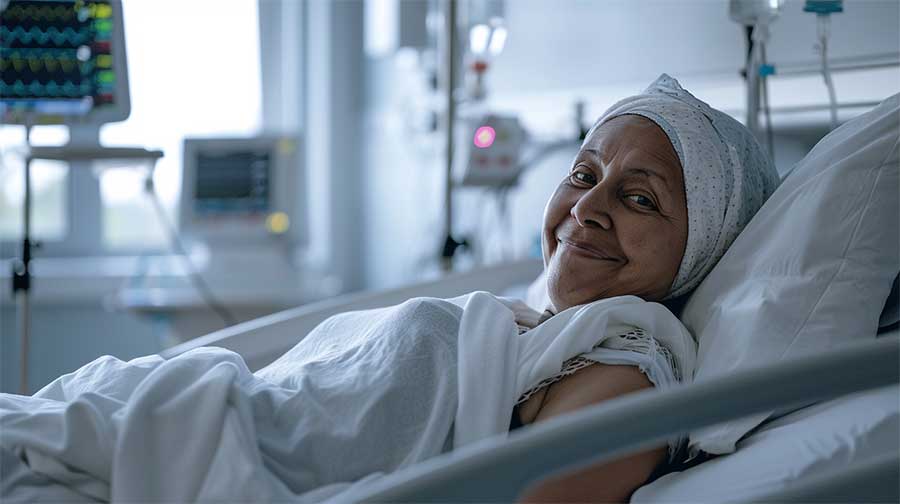

Companion Diagnostic play a vital role in solving the issue of drug side effects and involves analyzing specific biomarkers in patients to determine suitable treatments or to monitor side effects. This leads to a reduction of side effects due to unsuitable prescription of drugs, and patients will be provided better treatment tailored to their individual needs.
Irinotecan, an anticancer drug used globally to treat solid cancers , has reported numerous side-effects including death depending on patient genotypes, while Clopidogrel, a drug used for the prevention and treatment of cardiovascular diseases, also cause side effects. HeimBiotek developed SBDE-Plex™ Companion Diagnostics Kits based on SBDE™ PCR technology, to aid in managing side effects from Irinotecan and Clopidogrel.

UGT1A1 Assay Kit
Irinotecan
Anticancer drug, Irinotecan, is widely used to treat solid cancer recording annual sales of over 1 trillion Won, with 10,000 cases of the drug being administered in South Korea. However, the FDA, KFDA and European authorities recommend genotyping patients prior to Irinotecan prescription as it has been reported that patients with the genetic variation UGT1A1 are susceptible to side effects that may lead to death.
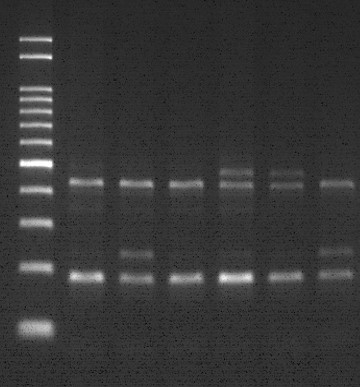
Dual-labeled probe used to accurately distinguish and analyze genotype UGT1A1 *6/*28.

CYP2C19 TotalAssay Kit
Clopidogrel (Plavix)
Clopidogrel is an antiplatelet drug, ranked 2nd in global sales. The extent of platelet aggregation inhibition varies among individuals administered Clopidogrel. Patients with a tolerance to Clopidogrel are at high risk for cerebral infarction and cardiovascular diseases such as myocardial infarction and acute thrombosis that may lead to death in extreme situations. Side effects have been reported in 56% of patients prescribed Clopidogrel and has been attributed to CYP2C19 loss of function alleles, thus emphasizing the importance of personalized medicine in antiplatelet therapy.
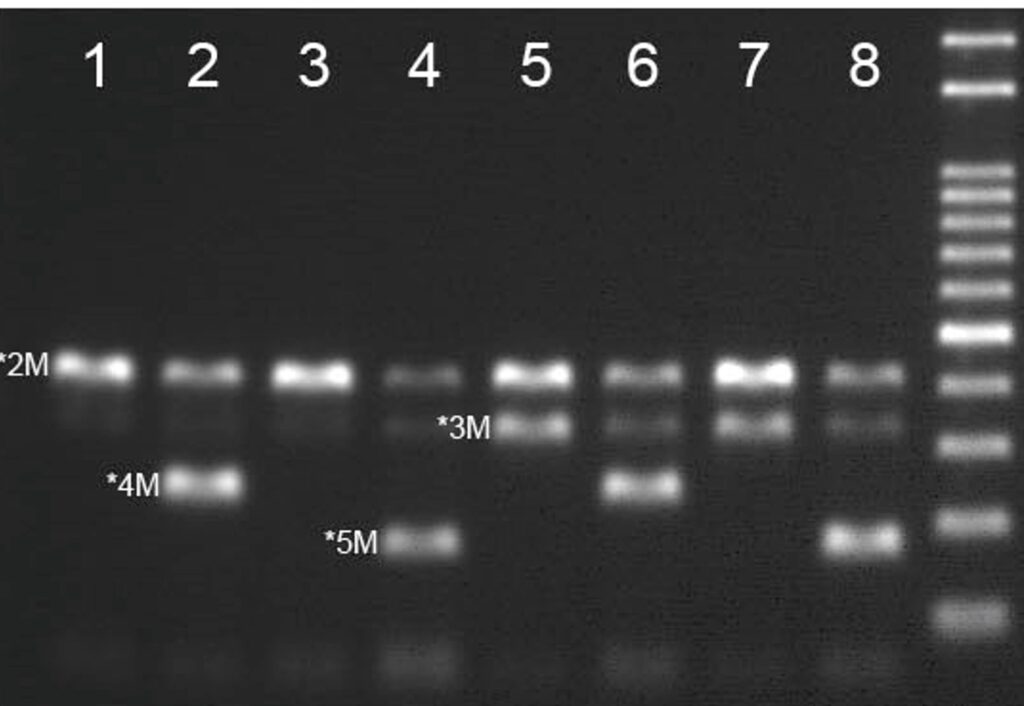
4 channel One-Step Real-Time PCR test to accurately distinguish and analyze genotype CYP2C19 *2/*3/*4/*5 without sample pretreatment.
Related Technology
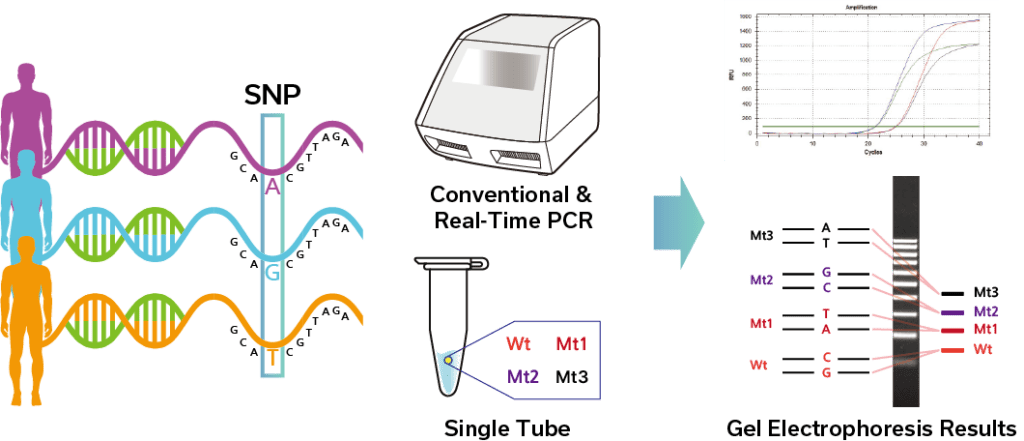

SBDE™ PCR Technology
SBDE™ (Specific Bi-Directional Extension) is a One-Step Multiplexing PCR technology with high sensitivity and specificity. It has the capability to differentiate between homozygosity and heterozygosity in Single Nucleotide Polymorphisms (SNPs) in addition to detecting Short Tandem Repeats (STR).
해당 제품의 관련한 기술인 HB SBDE™ 페이지로 이동합니다.

